Challenge
Cancer cells can become refractory to treatment causing therapy-resistant relapse
Breast cancer is the second leading cause of cancer-related death in women. While early-stage, non-metastatic disease is curable in ~70 to 80 % of patients, advanced breast cancer is considered incurable with currently available therapies.
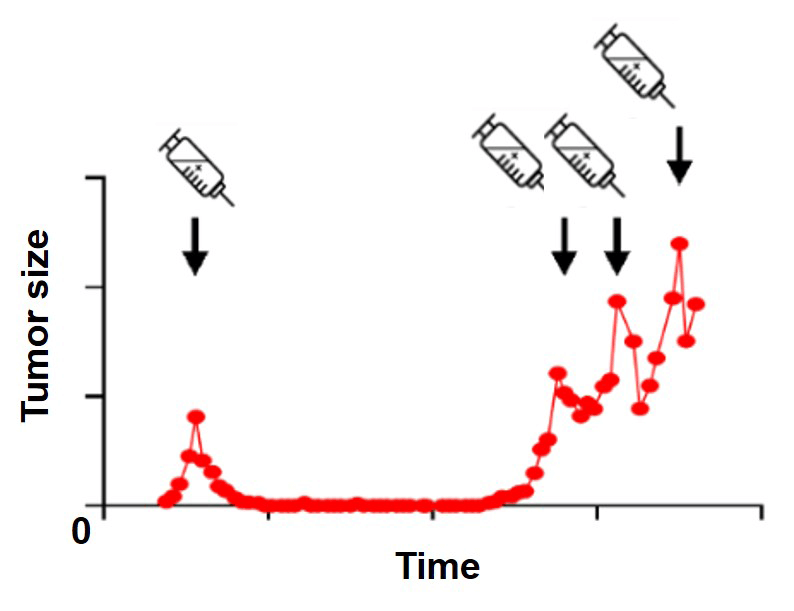
Treatment is initially effective in 90 % of primary breast cancers and 50 % of metastases. However, only about 10 to 25 % of breast cancer patients achieve complete response after systemic therapy. Even in these cases, if the entire cancer cell population is not eradicated, surviving cancer cells can persist.
The relapsing tumor may remain therapy sensitive and continue to respond to the initial treatment until mechanisms emerge causing stable drug resistance. Residual drug tolerant persister cells cause tumor reoccurrence and therefore are of investigational interest, but are challenging to detect.
Vision
The development of innovative multimodal imaging platforms will contribute to the detection, characterization, and eventual eradication of therapy resistant breast cancer
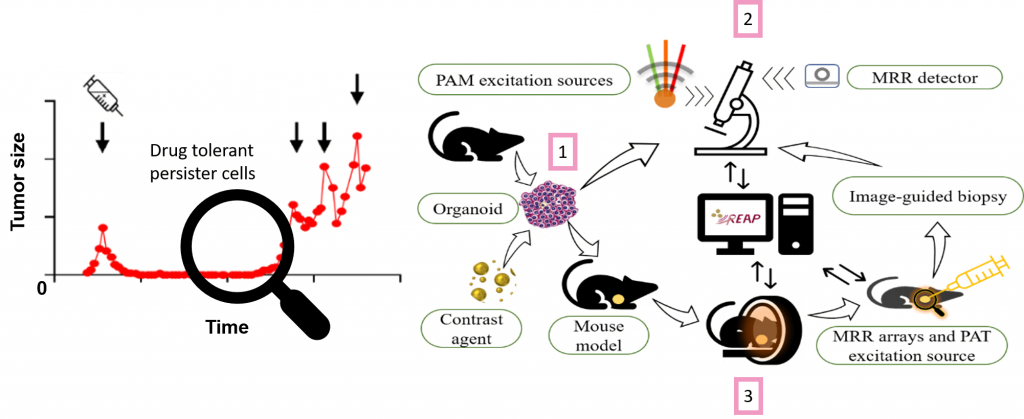
In REAP we envision to provide imaging systems useful across multiple scales to reveal drug tolerant persister cells in a preclinical setting. We will develop a triple modal two-photon laser scanning – optical coherence – photoacoustic microscopy for in vitro investigations as well as a dual modal optical coherence photoacoustic tomography for in vivo applications. Parallel developments in contrast agents based on biofunctionalized nanoparticles, new lasers, detector technology and real-time data handling aided by deep learning-based analysis will lead to the detection, characterization, and eventual eradication of persister cells.
Therapy resistant breast cancer will be modeled using genetically engineered organoid-based murine mammary tumors. Newly developed contrast agents will be assayed in vitro and in vivo by the 2-photon laser scanning photoacoustic optical coherence microscopy (2PLS-OC-PAM ) and the optical coherence photoacoustic tomography (OC-PAT) system, respectively, to detect and characterize rare cancer cells.
The triple modal two-photon laser scanning photoacoustic optical coherence (2PLS-OC-PAM) system will provide subcellular and cellular level resolution for up to 2 mm samples. In REAP a triple wavelength laser will be introduced for PAM alongside a new akinetic swept source with both interrogation function for PAM and swept source function for OCT to increase imaging speed. Micro-ring resonator-based (MRR) detector will be developed for superior sensitivity.
The optical coherence photoacoustic tomography (OC-PAT) system will be used for in vivo studies where increased imaging depth is required. Developments include optical parametric oscillator with high repetition rate and energy for PAT excitation, MRR arrays for real time detection, as well as dual-purpose lasers for all-optical detection photoacoustic interrogation and OCT. Real time image reconstruction algorithms for all the imaging modalities will also be implemented. Moreover, needle tracing algorithm will be implemented for image-guided biopsy. To close the circle, excised biopsies can be further analyzed with the microscopy system (2).
Latest News
June 30th 2025
The last consortium meeting of REAP was hosted by MUW on 10th and 11th June in Vienna. Despite numerous challenges, the consortium accomplished many tasks over the last 4.5 years. Three different types of lasers, including photoacoustic microscopy excitation lasers from Picophotonics Ltd., an OPO from InnoLas Laser GmbH operating at 1 kHz repetition rate, and an interrogation laser from LioniX International were demonstrated in this last consortium meeting. Along with the laser development, the Tampere University team and the AIT Austrian Institute of Technology team have achieved beyond the state-of-the-art performance of the micro-ring resonator. A dual modality optical coherence photoacoustic microscopy system and a 2 photon laser scanning microscopy system from Miltenyi Biotec have been successfully assembled and waiting to be combined into a triple-modality imaging system at Center for Cancer Research of MUW with the software package developed by Politecnico di Torino. Contrast agents developed by AIT Austrian Institute of Technology and the team from the Universidad de Santiago de Compostela have yielded promising results in the MUW labs. In the last few weeks of the project duration, a final sprint of imaging will be done in MUW, showcasing the use of REAP technologies in in vitro model imaging as well as mouse imaging. The whole consortium agreed to continue the collaboration after the lifetime of REAP and maximize the impact of the project by both commercial exploitation and scientific dissemination. It is an amazing journey of 4.5 years for research institutions, SMEs, and large enterprises to work together to push the front of biophotonics. The output of REAP is soon to be reaped.




Contact
-
Medical University of Vienna,
Spitalgasse 23, 1090 Vienna,
Austria, Europe - +43 1 40400 17150
- contact@projectReap.com